Physical & Chemical Properties of Wool Fibre | Part 04
Part 01: Wool Fibre | Wool Fibre Identification | Uses & Application of Wool fibre
Part 02: Wool Fibre | Manufacturing Process of Wool Fibre
Part 03:Wool Fibre Morphology | The Macro Structure of Wool
Physical & Chemical Properties of Wool Fibre
Wool Fibre
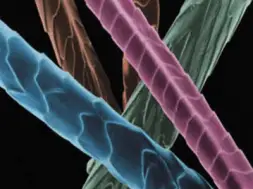
Wool is the natural protein fibre obtained from sheep and certain other animals, including cashmere from goats, mohair from goats, angora from rabbits, and other types of wool from camel. It is a multi-cellular, staple fibre. The density of fibre is 1.31g/cc, which is tends to make wool a medium weight fibre.
Chemical Composition of Wool Fibre
Wool is mainly a protein (keratin) fibre but it has also some other components, which are given below:
| Keratin | 33% |
| Saint |
28% |
| Fat |
12% |
| Mineral matter | 1% |
| Different impurities | 26% |
Elements in wool protein
The protein (keratin) of wool fibre consists of following basic elements:
| Carbon | 50% |
| Hydrogen |
12% |
| Oxygen |
10% |
| Nitrogen | 25% |
| Sulphur | 3% |
Chemical Bonds of Wool
The cross-linkages of wool polymers are hydrogen bonds, cysteine or Sulphur linkages, plus ion-to-ion bonds called salt bridges, peptide, ester and ether. There are also van der waals forces present in wool polymer.
Cysteine linkages contribute to: Strength, Lateral resistance, and react with – Alkali, Bleaches, Heat, Sunlight, “permanent set” agents, Moth – proofing agents.
Hydrogen bonds contribute to: Strength, Elasticity, “Temporary cell” and react with: Moisture deboned some intermolecular forces and decreases strength.
Salt bridge contributes to: Strength, and reacts with: Acids, Dyes.
Physical properties of wool
A physical property is any property that is measurable, whose value describes a state of a physical system. Wool fibre has some physical properties. Every of them is important.
|
SL. No. |
Parameters |
Properties |
|
1 |
Length |
60mm – 100mm sometimes it goes 250mm |
|
2 |
Diameter |
20µm t0 40 µm |
|
3 |
Strength |
1.0 – 1.7 gm/den |
|
4 |
Elongation |
25% – 35% under standard condition. |
|
5 |
Resiliency |
Excellent |
|
6 |
Hygroscopic (Moisture capacity 14 – 18%) |
Higher than other fibres.
|
|
7 |
Hand Feel |
Soft |
|
8 |
Specific Gravity |
1.32 and so fabrics fees lighter than cellulose. |
|
9 |
Dissolvability |
Wool dissolved in alkali solution |
|
10 |
Abrasion resistance |
Good |
|
11 |
Dimensional Stability |
Bad |
|
12 |
Color |
White to light cream in color. |
Wool insulates against heat, cold, and noise. It is healthy, water resistance, fire resistance, and naturally elastic, wears longer, versatile, resists static and dirt. Wool is easy to sew. It is comfortable. And Dyes beautifully.
Chemical Properties of Wool
Effects of alkali: Wool is easily and extremely vulnerable attacked by alkalis even by weak bases at low dilutions. alkaline solutions can open the disulphide cross-links of wool, white hot alkalis may even dissolve it. Wool dissolves when boiled in a 5% solution of sodium hydroxide. Caustic soda (NaOH) will completely damage wool when used hot or for a long period. Weak solutions of sodium carbonates can damage wool when used hot, or for a long period.
Effects of acids: Wool is more resistance to acids. Because they hydrolyze the peptide groups but leave the disulfide bonds intact, which cross link the polymers. Although this weakens the polymer system, it doesn’t dissolve the fibre.
- Wool is only damaged by hot sulphuric acid and nitric acid. Acids are used to activate the salt linkages in the wool fibre, making it available to the dye.
- Concentrated mineral acids will destroy wool if the fabric is soaked in it for more than a few minutes.
Effect of Bleaches: Bleaches that contain chlorine compounds will damage wool. Products with hypochlorite will cause wool to become yellow and dissolve it at room temperature. Various forms of chlorine are used to make ‘unshrinkable wool’, by destroying the scales. This wool is weaker, less elastic and has no feeling properties. Wool is irreversibly damaged and colored by dilute oxidizing bleaches such as hypochlorite.
Bleaches containing –
- Hydrogen peroxide
- Sodium perborate
- Sodium peroxide
- Potassium permanganate
Won’t harm wool and are safe to use for stain removal.
Reduction: Under controlled conditions, reducing agents can be used to partially reduce the wool.
Effect of Sunlight: Wool will weaken when exposed to sunlight for long periods. The ultraviolet rays will cause the disulfide bonds of cysteine to break, which leads to photochemical oxidation. This will cause fibre degradation and eventual destruction. Wet fabrics exposed to ultraviolet light are more severally faded and weakened than dry fabrics. Wool is attacked by short wavelength (300 – 450 nm) UV light, causing slow degradation and yellowing.
Effect of perspiration: As already stated, wool is easily deteriorated by alkalis and therefore perspiration which is alkaline will weaken wool as a result of hydrolysis of peptide bonds and amide side chains. Perspiration in general will lead to dis coloration.
Effect of Water: Wool absorbs moisture (hygroscopic). Wool can absorb about 30% of water vapor without feeling wet. The moisture is released only slowly. In spite of the strong affinity for water of the fibre interior its surface is water repellent (hydrophobic) because it is covered by an extremely thin skin. This skin causes liquid water to roll up into droplets whilst allowing the passage of water vapor.
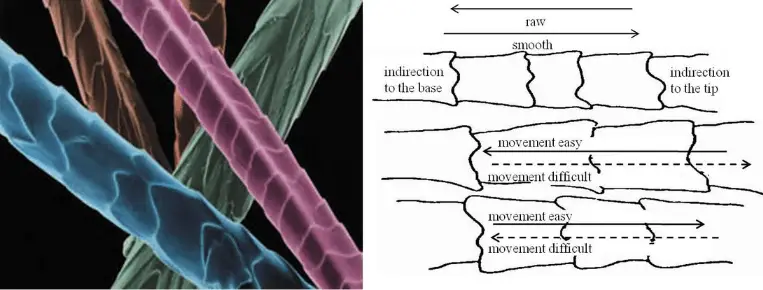
Prolonged boiling will reduce luster and promote felting. The heat makes the fibre more elastic and plastic which makes it easier to move and entangle itself with other fibres.
Part 01: Wool Fibre | Wool Fibre Identification | Uses & Application of Wool fibre
Part 02: Wool Fibre | Manufacturing Process of Wool Fibre
Part 03:Wool Fibre Morphology | The Macro Structure of Wool
(3506)