Dyeing Of Polyester Fabric With Disperse Dyes
Dyeing Of Polyester Fabric With Disperse Dyes (Carrier Method).
Objects :
- To dye a material practically.
- To observe the practical advantages and disadvantages of this dye.
- To compare the theoretical idea with practical experiment.
Introduction :
Dyes are coloured, unsaturated organic chemical compounds capable of giving colour to a substrate (a textile), i.e. colouring or dyeing it.
The term “disperse dye” have been applied to the organic colouring substances which are free from ionizing groups, are of low water solubility and are suitable for dyeing hydrophobic fibres. The dye has derived its name for its insoluble aqueous properties and the need to apply it from an aqueous dispersion. Of all the dyes, they are of the smallest molecular size.
The dyeing of hydrophobic fibres like polyester fibres with disperse dyes may be considered as a process of dye transfer from liquid solvent (water) to a solid organic solvent (fibre).
Disperse dyes are added to water with a surface active agent to form an aqueous dispersion. The insolubility of disperse dyes enables them to leave the dye liquor as they are more substantive to the organic fibre than to the inorganic dye liquor. The application of heat to the dye liquor increases the energy of dye molecules and accelerates the dyeing of textile fibres.
Heating of dye liquor swells the fibre to some extent and assists the dye to penetrate the fibre polymer system. Thus the dye molecule takes its place in the amorphous regions of the fibre. Once taking place within the fibre polymer system, the dye molecules are held by hydrogen bonds and Van Der Waals’ force.
Carriers :
It has been established that certain hydrocarbons, phenols, amino acids, amides, alcohols, esters, ketones, nitriles etc. accelerate the rate of dyeing polyester fibre with disperse dyes from aqueous medium at temperature up to 100°C. These dyeing assistants alter the dispersing properties of the dyes and the physical characteristics of the fibre so that more dye can be transferred from the dye bath to the fibre. These are called carriers and are necessary for dyeing polyester fibres at the normal pressure and temperature below 100°C to increase the dyeing rate and to permit dye migration within the fibre. Level dyeing of disperse dyes depend on the migration power of the dye which is affected by nature and amount of carrier, dyeing time, temperature and the shade.
Mechanism of Carrier Action :
In carrier method of polyester dyeing, carrier is used. Carriers swell the fibre and ultimately cause relaxation. They may operate by opening up the internal fibre structure and allow the dye molecules to diffuse more rapidly. They act as molecular lubricants reducing inter-molecular forces operating in the fibre, thereby following the dye molecule to force its way in. Its action may be described as below:
- It creates dye film on fibre surface.
- Carrier takes dye inside the fibre from dye carrier association.
- It increases the solubility of dye in the dye bath.
- Carriers penetrate inside the fibre polymer chain and thereby reduce inter-chain attraction. Thus polymer chains become movable and so dye molecules may enter the polymer system of fibre.
- It increases fibre swelling.
- The absorbed carrier increases the rate of dye uptake by creating liquid co-fibre.
- It increases the absorbency power of fibre.
- It lubricates the thermally agitated fibre molecules.
- 2-10 gm/lit carrier is used depending on material and liquor ratio and depth of shade.
- The automatic portion of carrier is postulated to have Van Der Waal’s force and attraction for hydrophobic group of it attracts water.
- With increasing molecular weight the carrier efficiency also increases up to a certain limit.
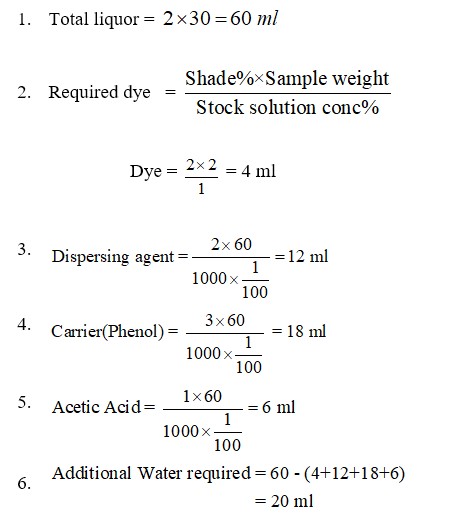
Dye = 2% on the weight of fabric
Dispersing agent = 2 g/L
Carrier (Phenol) = 3 g/L
Acetic acid = 1 g/L
M: L = 1:30
Sample weight : 2 gm polyester fabric.
Calculation:
Procedure :
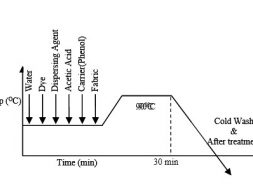
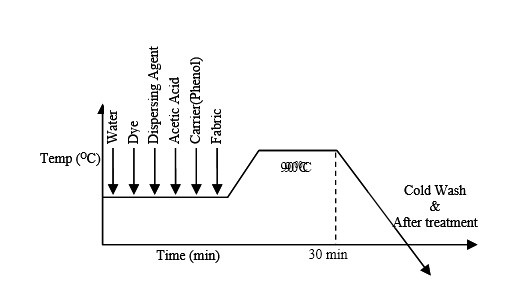
According to dyeing curve at first auxiliaries and water are added in the dye bath. And it is kept for 5 minute. Then material, dye is added respectively. Then after 10 minutes salt is added. After adding salt dye bath is heated to 400-500C kept for 20-30 minutes. This is the exhaustion period of dyeing. Then alkali is added in the dye bath. After adding alkali the dye bath is heated for 40-60 minute at 50-600C. This is the fixation period.
After treatment :
- The material is treated with a 1g/L soap solution, which removes the unfixed dye from fabric surface, and makes the surface clean.
- Material is treated with a hot water bath.
- Material is treated with a cold-water bath.
- Finally the material is dried in a drier.
Remark :
The sample of the dyed material seems to have correct shade and it shows good fastness especially wash fastness. So it is proved that maintaining proper temperature and time does the experiment.
(7513)