Introduction to Sulfur Dyes Part 1
Introduction to Sulfur Dyes
Sulfur dyes are complex heterocyclic molecules or mixtures formed by melting or boiling organic compounds containing amino or nitro groups with Na-polysulphide and Sulfur. Sulfur dyes are so called as they all contain Sulfur linkage within their molecules.
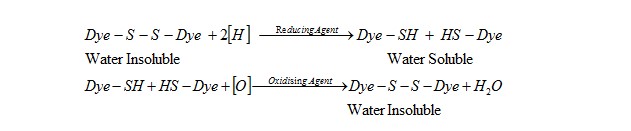
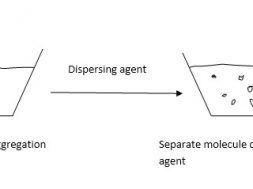
Sulfur dyes are highly coloured, water insoluble compounds and have to be converted in to water soluble substantive forms (lucoforms) before application to the textile materials. This conversion is carried out by a treatment with a reducing agent like dilute aqueous Na2S. Since this lucoform of Sulfur dye is substantive to cellulosic materials. They are absorbed on the fibre surface. Then they are reconverted original water insoluble form of dye by oxidation. This oxidation is carried out by “airing” (exposure to air) or by using an oxidizing agent like Na-dichromate (Na2Cr2O7).
The reducing agents converts the “S” in dye in to –SH group and the Sulfur linkages. Then inside the material the thiols containing –SH groups are oxidized & thus reconverted to original form of dye.
Sulfur gives best result (Bright Tone) when they are used to produce black, Black & brown shades but red shades cannot be obtained by Sulfur dyes.
History of Sulfur Dyes
The history of Sulfur dyes may be summarized as below:
- The first Sulfur dyes where made in 1873 heating saw dust, caustic soda and Sulfur. It occurred by chance when a reaction vessel containing Na2S was leaking and the saw dust was used to wipe the solution coming out. Later a cotton fabric come in contact with this contaminated sawdust and become stained.
- The real pioneer of Sulfur dyes was vidal who produce vidal black (Name of Sulfur dye) by fusing para-phenylene diamine with Na2S & Sulfur in 1893.
- In 1897 Kalischer produced Immedial Black FF by heating 2, 4-dinitro-4-dihydroxy diphenylamine with Na-poly sulphide.
- In 1896 Read Holliday introduced a range of grey, brown and black Sulfur dyes by the action of Sulfur, alkali sulphides and many organic compounds.
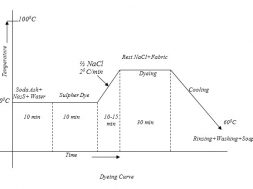
Method of manufacture of Sulfur dye
Trade Names of Sulfur Dyes
|
Trade Names |
Name of Manufacturer |
Country of origin |
|
Calcogen |
Dyes Dept. American Cyanamid Co. |
USA |
|
Pyrogene |
Ciba |
Switzerland |
|
Thional |
Sandoz |
Switzerland |
|
Solfo |
ACNA |
Italy |
|
Sulfogene |
Du pont |
USA |
|
Thional |
Imperial |
UK |
|
Mitsui Sulfur |
Mitsui Chemicals Ind. Co. Ltd |
Japan |
Characteristics of Sulfur Dyes
The main properties and characteristics features of Sulfur dyes are mentioned below:-
- Sulfur dyes have Sulfur linkage within their molecules.
- Sulfur dyes are highly colouerd water insoluble dyes. Some dyes are partially soluble in water.
- They have no direct affinity towards cellulosic fibres. To make them substantive they are to be converted in to soluble lucoform by treating them with reducing agents (Like dilute Na2S solution)
- Sulfur dyes have good light fastness with rating about 4. This light fastness may be improved by an after treatment with metallic salt.
- These dyes have excellent wash fastness with rating about 3-4. This good wash fastness is due to its larger molecular size & insolubility in water.
- They are not applicable to wool due to strong alkaline condition.
- They are exclusively amorphous, few of them show crystallinity.
- Important for producing a wide range of shades on a varity of cotton and rayon.
- Sulfur dyes are suitable for heavy & durable shades
- Available in powder and soluble form
- Sulfur dyes are cheap & easy to manufacture.
- Heat and chemical resistance of Sulfur dyes are moderate to good. They have poor fastness to chlorine and are not applied to goods which are bleached with hypochlorite.
Features of Sulfur Dye
- Amorphous Colloidal materials.
- High molecular weight with various composition
- Complex molecular structure –heterocyclic molecules containing Sulfur linkage.
- Decomposed by acids, with the liberation of H2S.
- Characterized by thiozine ring, containing Sulfur atom.
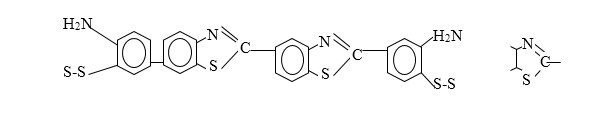
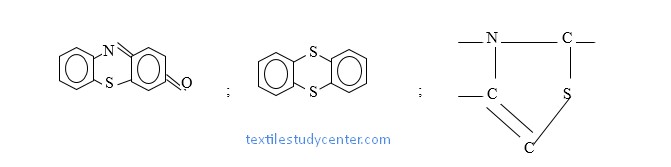
Chemical Structure of Sulfur Dye
Sulpher dye contain Sulpher atom in their molecule and is characterized by the thiozine ring
A portion of Sulpher dye molecule is shown below:
The structure formula is incomplete because the complete composition and structure of Sulpher dye is mot known.
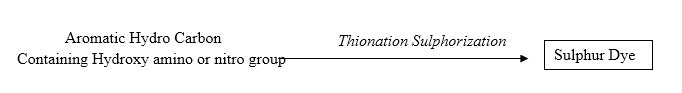
Chemical Nature of Sulfur Dyes
The manufacturing method of Sulfur dyes may be shown as below:-
This reaction is carried out in a closed vessel in the presence or absence of solvents. For this (Thionisation/Sulphorisation) purpose Sulfur or Na- polysulphide is used. The features of the products of thionation are controlled by organic compounds, conditions of reaction (time, temp etc). Condition of separating dyes from reaction mixture etc.
After the reaction is over the dye is precipitated acidification or oxidation or both.
Generally Sulfur dyes are marketed in forms of powder pastes or liquid solutions. The dyes are amorphous colloidal materials of high molecular weight and variable composition. Their exact chemical composition is not yet established. However they are complex in structure. Some amorphous present in Sulfur dyes are as below:
Chemistry of dyeing with Sulfur Dye
The Sulfur dyes contain Sulfur linkage within their molecules. They are insoluble in water but can be made soluble in water by treating them with reducing agents. This also makes them substantive towards cellulosic fibres. Na2S acts as reducing agent that breaks the Sulfur linkage and break down the longer molecules in to simple components which can penetrate the material (fiber/fabric) surface easily.
This thios containing the –SH groups are readily oxidized by the action of atmospheric O2 or any other oxidizing agents. This reconverts the water soluble luco form of Sulfur dye in to previous water insoluble form which has a very good wash fastness property.
Part 02 :Classification of Sulpher Dyes | Sulfur dyeing methods | oxidizing and reducing agent for sulfur dyeing | Reducing Steps of Sulfur Dyes |Oxidation Step of Sulfur Dye| Dyeing of Cellulosic Fibres with Sulfur Dyes |Typical Recipe for sulfur dyeing | Good Preparation for sulfur dyeing | Dye solution preparation or Reducing Step for sulfur dyeing | Dyeing with sulfur dye |Oxidation of sulfur dye |After treatment for sulfur dye | Precaution in the sulfur dyeing process|Control of Dyeing for sulphar dye | Topping of Sulpher Dyes |Improving of Fastness Properties of sulfur dye
Part 03 :Defects of sulfur Dyeing | Bronziness of Shades | Causes of Bronziness of Shades | Remedies of Bronziness of Shades | Sulfur Black Tendering | Causes of Sulfur Black Tendering | Remedies of Sulfur Black Tendering | Stripping of Sulfur dyes | Uses of Sulfur Dye | S-Radical in Sulfur dye | Colors/Shades found from Sulfur dyes | Causes for the Popularity of Producing Black Shades with Sulfur Dyes | Comparison between Sulfur & Vat Dyes | Reasons for why so called
Reference
- Broadbent A D 2001 Basic principles of textile coloration Society of Dyers and Colourists p 566
- Shenai V A Chemistry of Dyes and Principles of dyeing
- Carr C M 1995 Chemistry of the Textiles Industry (Dordrecht: Springer Netherlands)
- Chakraborty J N Fundamentals and Practices in Colouration of Textiles
- John Wiley & Sons Inc 2000 Kirk-Othmer Encyclopedia of Chemical Technology (Hoboken, NJ, USA: John Wiley & Sons, Inc.)
- Ühnel G E R D K, Aktiengesellschaft B, Olff J O W, Ag B, Uppert G Ü R, Aktiengesellschaft B, Chmitt M I S, Aktiengesellschaft B, Etired C H H E I D R, Ag C, Etired M A X H Ü R, Aktiengesellschaft H, Etired H E L E R and Aktiengesellschaft B 2016 Textile Dyeing , 2 . Dyeing of Cellulose Fibers 1–27
- Hunger K 2003 Industrial Dyes: Chemistry, Properties, Applications
- Rahman M M Wet Processing 1
- Iqbal M 2008 Textile Dyes 167
- ARNOLD R. LANG DYES AND PIGMENTS: NEW RESEARCH
(4117)