Absorption of IR radiation method
Methods of investigation of fiber structure
There are many methods for measuring of fiber structure. Such as….
⦿ Absorption of IR radiation method
⦿ X-ray diffraction method
⦿ Physical properties
⦿ Chemistry properties – its preparation, composition, molecular formula and reactions
⦿ Thermal properties
⦿ Optical properties
⦿ Optical microscopy
⦿ Electron microscopy and electron diffraction
⦿ Nuclear magnetic resonance
⦿ Raman scattering of light
⦿ Density
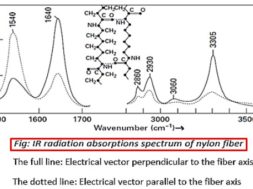
Absorption of IR radiation method
Infra-red (IR) spectroscopy is one of the most common and widely used spectroscopic techniques. When electromagnetic waves interact with matter, they are scattered and absorbed. Infrared spectroscopy, radiation with wavelengths between 1 -15 μm is absorbed at certain characteristic frequencies, which yield structural information.
By using an infrared spectrometer, the variation in absorption can be found and plotted against wavelength or the wavenumber.
Wavenumber = 1 / wavelength in centimeters
The wavenumber at which absorption takes place depends on
❶ The nature of the two atoms and
❷ The bond between them.
❸ The other groups in the neighborhood
The peaks occur where the frequency of the electromagnetic waves corresponds with the natural frequency of vibration between two atoms in the material.
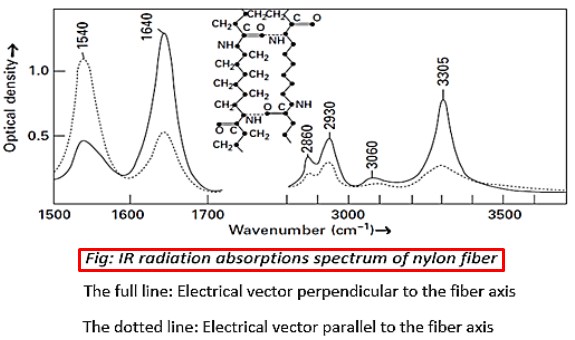
The peaks are due to:
1540 cm-1 uncertain, involves (—NH) and neighboring bonds
1640 cm-1 stretching of C=O
2860 and 2930 cm-1 stretching of =CH2
3305 cm-1 stretching of N—H
Advantages of IR
⦿ It can be used for identification of the presence of certain groups in the molecules
⦿ It can be used to investigate the degree of orientation of fiber.
⦿ It can be used to determine the amount of water in fibers.
⦿ It can be used to determine the α & β molecular configuration of fiber.
⦿ It gives information of both crystallinity & non-crystallinity of fibers.
IR active and inactive compound
IR Active compound:
⦿ If the vibration transition in molecule is capable of change in dipole moment, so the molecule is said to be IR active.
⦿ Asymmetrical stretching/bending and internal rotation changes the dipole moment of a molecule. Asymmetrical stretching/bending are IR active.
⦿ Homo-nuclear atoms are IR inactive. Example: C=0, N-H, O-H etc.
IR Inactive Compounds
⦿ If the vibration transition in molecule does not produce a change in dipole moment, so the molecule is said to be IR inactive.
⦿ So the symmetric compound is inactive in IR
⦿ Hetero-nuclear atoms are IR active if their vibrations result in a net dipole moment. Example: C=C, Cl2,H2 etc.
Note: There is slight positive & negative charge on its component atoms & changed the distance between charges atoms called change in dipole moment.
Requirements of IR Radiation absorption
Correct wavelength of radiation: A molecule can absorb IR radiation, if the natural frequency of vibrations of some part of a molecule is equal to the frequency of incident radiation.
Change in dipole moment: A molecule can only absorb IR radiation when its absorption causes a change in its electric dipole.
Molecular vibrations
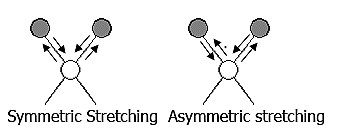
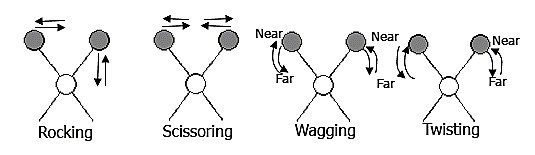
The positions of atoms in a molecule are not fixed; they are subject to a number of different vibrations. Vibrations fall into the two main categories of stretching and bending.
❶ Stretching: Change in inter-atomic distance along bond axis.
❷ Bending: Change in angle between two bonds. There are four types of bend:
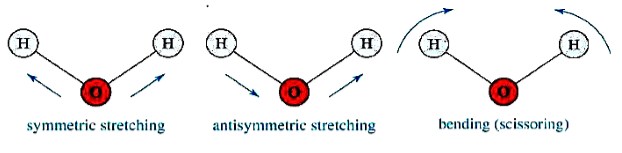
Molecular vibration modes of H2O
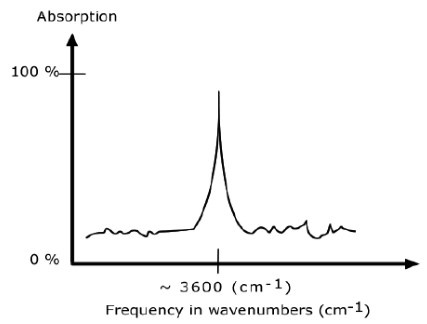
An IR spectrum in absorption mode
The IR spectrum is basically a plot of transmitted (or absorbed) frequencies vs. intensity of the transmission (or absorption). Frequencies appear in the x-axis in units of inverse centimeters (wavenumbers), and intensities are plotted on the y-axis in percentage units.
The graph above shows a spectrum in absorption mode.
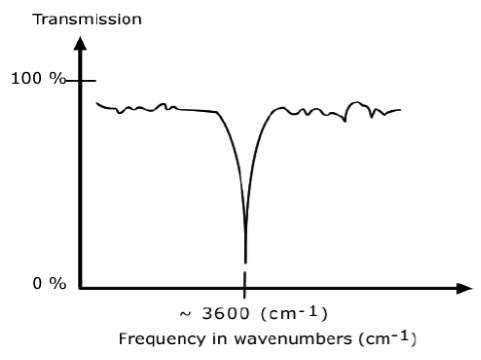
An IR spectrum in transmission mode
This is the most commonly used representation and the one found in most chemistry and spectroscopy books. Therefore we will use this representation.
The graph above shows a spectrum in transmission mode.
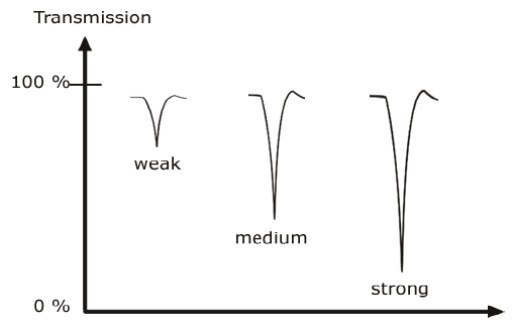
Classification of IR bands
IR bands can be classified as strong, medium, and weak, depending on their relative intensities in the infrared spectrum.
A strong band covers most of the y-axis.
A medium band falls to about half of the y-axis,
A weak band falls to about one third or less of the y-axis.
(1819)