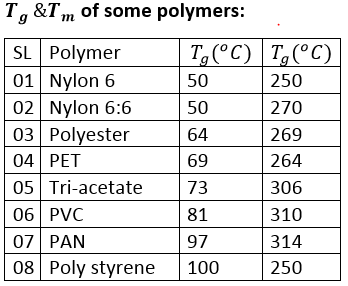
Thermal Properties of Polymers
Thermal Properties of Polymers :
Thermal property:
The property which is shown by a textile fiber when it is subjected to heating is called thermal property.
Thermal property is included:
- Thermal conductivity
- Melting transition temperature
- Glass transition temperature
- Heat setting
- Thermal expansion
- Heat of wetting or heat absorption
- Flammability
Thermal conductivity: Thermal conductivity is the rate of heat transfer in degree along the body of textile fiber by conduction. (The thermal conductivity of textile fiber depends to a much greater extent on the air entrapped within it than on the fiber conductivity)
- Higher thermal conductivity indicates that the fiber is more conductive.
- In winter session, lower conductivity fiber dresses are more suitable to wear.
Factors affecting thermal conductivity
Thermal conductivity mainly depends on following matters.
- Temperature: The temperature dependence of thermal conductivity for amorphous polymers increases gradually in the glassy resign and decreases slowly or remains constant in the rubbery region. For crystalline polymers, thermal conductivity decreases steadily with the increase in temperature below . At temperature above , it behaves in a similar way as amorphous polymers.
- Degree of crystallinity: Thermal conductivity depends on the degree of crystallinity; a polymer with highly crystalline and ordered structure will have higher conductivity than amorphous polymer.
- Density of polymer: The thermal conductivity increases with the increasing of density of polymer.
- Orientation of chain segments: Thermal conductivity of polymer is highly dependent on the polymer chain segment orientation. This is because thermal energy transports more efficient along the polymer chain. Crystalline polymers have highly oriented chain segments, and therefore have higher thermal conductivity than amorphous polymers.
- Structure: The cell size of foamed polymer may also have an effect on thermal conductivity. Smaller foam cell size tend to lower thermal conductivity. Most foamed polymers have thermal conductivity values in the order of , which is about 10 times less than the same polymers.
Why woolen dress are more suitable than cellulosic dresses?
The protein fiber (wool) has a lower thermal conductivity than cellulosic fiber. That’s why the woolen (protein) dresses are more suitable than cellulosic/synthetic dresses.
Why synthetic dresses are not suitable in summer and winter season?
We know that lower thermal conductivity fiber dresses are more suitable to wear. The value of thermal conductivity of synthetic fiber (polyester, nylon etc.) is higher than natural fiber (cotton, silk, wool etc.). As a result the synthetic dresses are more conductive, hence rate transfer of heat along a body is very high. So, in summer season, atmospheric heat is easily felt and in winter season, low temperatures of air is also effect on the body by synthetic fiber dresses. That’s why, the synthetic dresses are not suitable in summer and winter season.
Why silk fiber is warm in winter and cool in summer?
Thermal conductivity of the silk fiber is expressed in terms of the rate of heat conductance (thermal conductivity co-efficient).The thermal conductivity is poor and the specific heat is higher than other natural fibers and thus silk is warm in winter and cool in summer. This is due to the fact that the cocoon filament is a porous fiber having numerous vacuum spots capable of accommodating large quantity of air.
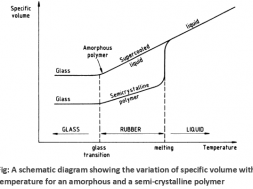
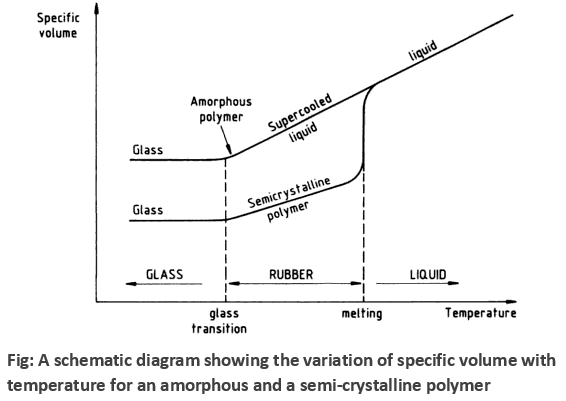
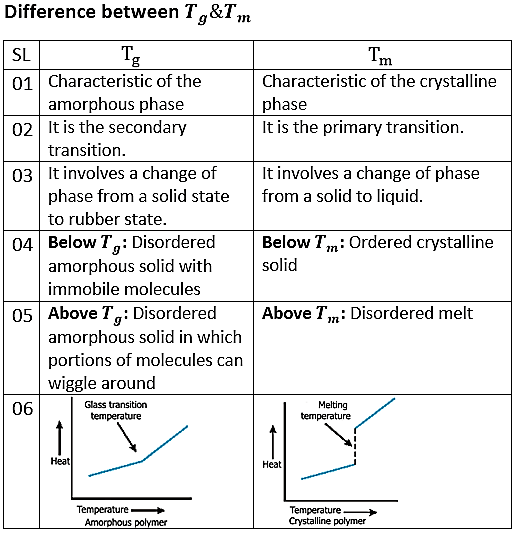
In the amorphous region of the polymer, at lower temperature, the molecules of the polymer are in, say, frozen state, where the molecules can vibrate slightly but are not able to move significantly. This state is referred as the glassy state. In this state, the polymer is brittle, hard and rigid analogous to glass. Hence the name glassy state. The glassy state is similar to a supercooled liquid where the molecular motion is in the frozen state. The glassy state shows hard, rigid, and brittle nature analogous to a crystalline solid with molecular disorder as a liquid. Now, when the polymer is heated, the polymer chains are able to wiggle around each other, and the polymer becomes soft and flexible similar to rubber. This state is called the rubbery state. The temperature at which the glassy state makes a transition to rubbery state is called the glass transition temperature Tg . Note that the glass transition occurs only in the amorphous region, and thecrystalline region remains unaffected during the glass transition in the semi-crystalline polymer.
Glass transition temperature ( Tg ) :
When the polymer cools and the temperature lowers, the mobility in the amorphous regions of the polymer decreases. The lower the temperature, the stiffer the polymer becomes until a point of transition is reached. This transition is called the second order transition temperature or the glass transition temperature. It is denoted by Tg and the range of Tg for linear fiber is like between -200 to 300oC
Factors influence the Tg:
- Flexibility of chain bond decrease the value of Tg.
- Flexibility of side group decrease the value of Tg.
- Polarity of side groups increase the value of Tg.
- Bulky of side groups increase the value of Tg.
- Composition of ring structure in the molecule chain raises the value of Tg.
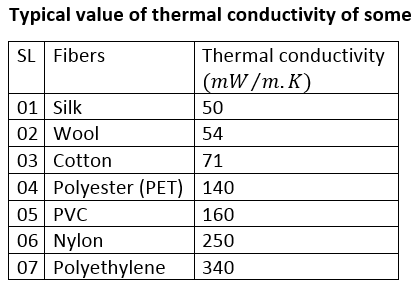
- Random co – polymer has a lower value of Tg than homo-polymer.
- Tg increases with moleclues weight up to 20,000.
GLASS TRANSITION TEMPERATURE
The mechanical properties of polymers radically change at the glass transition temperature Tg; molecular motion is the underlying cause of the change. Below Tg there is no translational or rotational motion of the atoms that make up the polymer backbone, but these motions are present above Tg. Below Tg, polymers are relatively hard, inflexible and brittle, whilst above it they are soft and flexible. The terms glassy, and rubbery or leathery are used to describe properties in the two temperature regions.
Melting temperature
The temperature at which the fiber melt completely is called first order transition temperature or melting temperature. It is denoted by Tm.
- At melting temperature fiber losses its density and convert it into a viscos liquid.
- At melting temperature fiber also losses its strength and some molecules weight.
Melting point depends on:
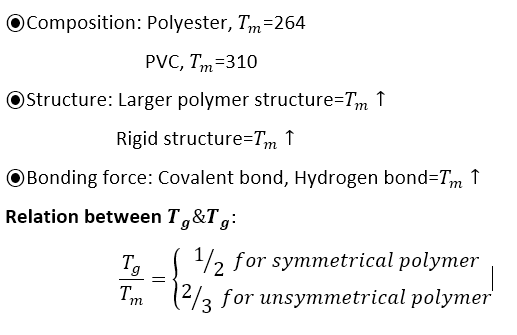
Specific volume vs Temperature curve with Tg and Tm for amorphous and semi-crystalline polymer
Fig. shows the decrease of specific volume with temperature for amorphous and semi-crystalline polymers. As a semi-crystalline polymer is cooled from its liquid state through the melting temperature, its specific volume drops abruptly reflecting the closer and regular packing of molecules into crystalline structures. If the polymer is amorphous then no abrupt drop in specific volume will be observed at the melting temperature, it will simply continue to decrease linearly with decreasing temperature until the glass transition temperature is reached. Both amorphous and semi-crystalline polymers are mechanically rubbery at temperatures between the melting and glass-transition temperatures. In the rigid glassy state below the glass transition temperature the specific volume of both amorphous and semi-crystalline polymers continues to decrease, but at a slower rate.

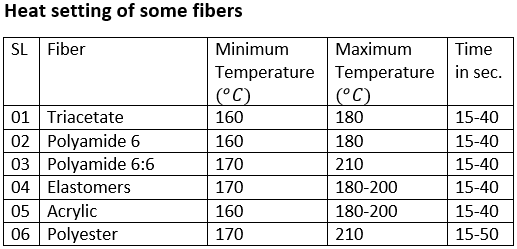
Heat setting:
Heat-setting is a heat treatment by which shape retention, crease resistance, resilience and elasticity are imparted to the fibers.
®Heat setting is done before the melting temperature and dyeing is done above glass transition temperature If the fabric is dyed before heat setting we will not the desired result. Fabric will be deformed.
Objects of heat setting
- To impact dimensional stability.
- To remove shrinkage of fabric.
- To decrees crease resistance.
- To increase elasticity and resiliency.
Disadvantages of heat setting
- Fiber become very stiff.
- Crystallinity increase but dye takes decrease.
- Fiber color may be change.
Types of heat-setting
There are three types of heat-setting
- Temporary heat setting
- Semi-permanent heat setting
- Permanent heat setting
Temporary heat setting: This type of setting is destroyed by regular use of the materials. For examples- a stream pressed cotton textile.
Semi-permanent heat setting: This type of heat setting materials is raised above it’s Tg and then set into a new form.the setting is lost when the mlts is subjected to severe condition of use. For example, hot washing or steaming of materials above Tg .
Permanent heat setting: This type of heat setting involves change of the material in such a way that it would not reverse till the materials is destroyed by taking it above its melting point. For example, heat setting to develop new crystallites.
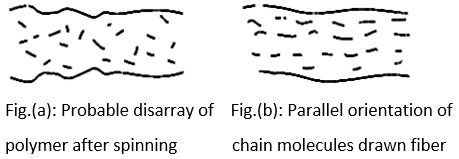
Thermal behaviour of synthetic fibers or structural changes due to heat setting
Synthetic fibers, mainly polyester and nylon, consist of long chain molecules and are held together by inter-chain bonds. Fig. (a) shows how the chains are irregularly distributed at random in single fibers immediately alter spinning. The fibers are then stretched several times their original length to impart desirable properties to the fiber and this causes orientation of chain molecules parallel to the fiber axis [Fig. (b)].
Why nylon tends to be heat set at a higher temperature than polyester?
There is an important difference between the behaviour of the two common polyamides (nylon 6 and nylon 6.6) and polyester, because of their different behaviour towards water. Polyester is non-absorbent, so the heat setting behaviour is not affected by water. However, nylon will absorb sufficient water to obtain a temporary set that is based on hydrogen bonding and is destroyed on boiling in water. The consequence of this is that to obtain a permanent set on nylon, the water has to be removed from the fiber so that crystallization can take place. Therefore nylon tends to be heat set at a higher temperature than polyester.
Thermal expansion: Thermal expansion can be measured by co-efficient of thermal expansion and which is defined as the fraction increase in length of a specimen to rise in temperature by 1oC.
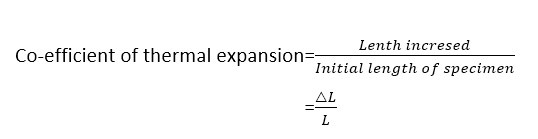
Heat of wetting: When a textile fiber absorbs moisture or water it gives off some amount of heat which is called heat of wetting OR heat of absorption.
®Head of absorption resulting from changes in moisture region rather than the thermal conductivity.
®If 1gm of dried textile fiber is completely wetted then heat is calorie/gm is involved which is known as heat fiber.
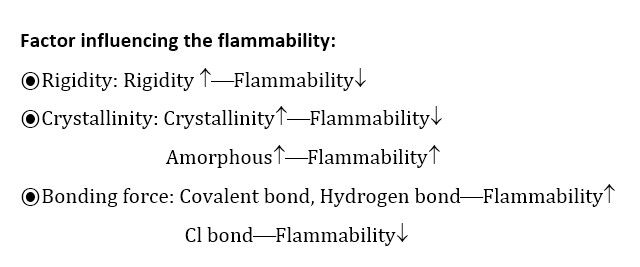
Flammability: Flammability is defined as how easily smoothing will burn or ignite, causing fiber or combustion. It is measured by passing a mixture of oxygen and nitrogen over a bearing specimen, and reducing the oxygen level until a critical level is raised.
(4396)