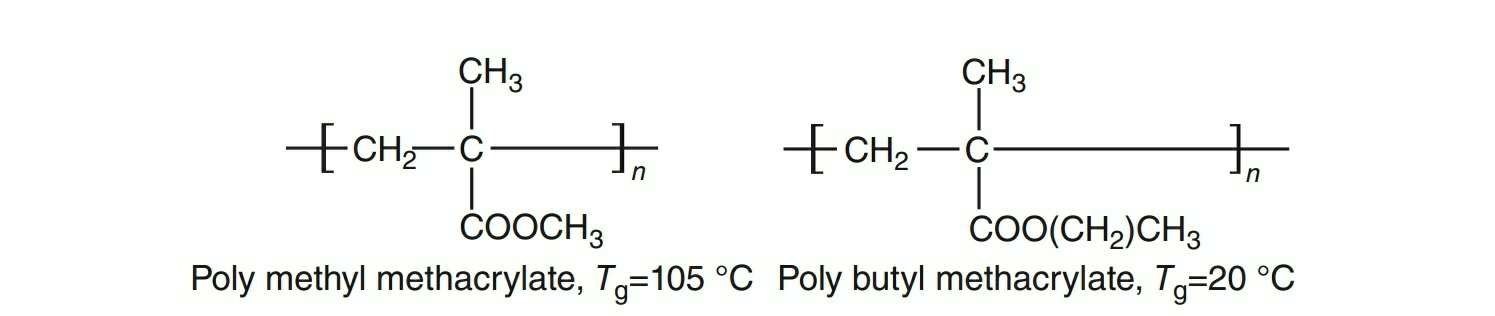Thermal Properties of Polymers | Melting Point and Glass Transition Temperature
Thermal Properties of Polymers
In the amorphous region of the polymer, at lower temperature, the molecules of the polymer are in, say, frozen state, where the molecules can vibrate slightly but are not able to move significantly. This state is referred as the glassy state. In this state, the polymer is brittle, hard and rigid analogous to glass. Hence the name glassy state. The glassy state is similar to a super cooled liquid where the molecular motion is in the frozen state. The glassy state shows hard, rigid, and brittle nature analogous to a crystalline solid with molecular disorder as a liquid. Now, when the polymer is heated, the polymer chains are able to wiggle around each other, and the polymer becomes soft and flexible similar to rubber. This state is called the rubbery state. The temperature at which the glassy state makes a transition to rubbery state is called the glass transition temperature Tg . Note that the glass transition occurs only in the amorphous region, and thecrystalline region remains unaffected during the glass transition in the semi-crystalline polymer.
Melting Point and Glass Transition Temperature
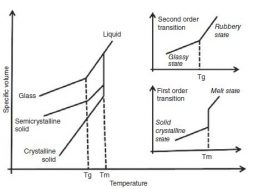
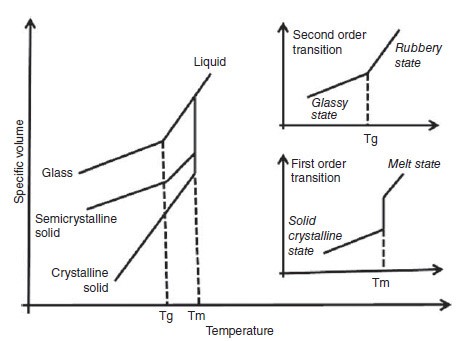
The glass transition temperature is the property of the amorphous region of the polymer, whereas the crystalline region is characterized by the melting point. In thermodynamics, the transitions are described as first and second order transitions. Glass transition temperature is the second order transition, whereas the melting point is the first order transition (see Fig. 1). The value of glass transition temperature is not unique because the glassy state is not in equilibrium. The value of glass transition temperature depends on several factors such as molecular weight, measurement method, and the rate of heating or cooling.
Approximate values of glass transition temperatures of some polymers are listed in Table below.
The semi-crystalline polymer shows both the transitions corresponding to their crystalline and amorphous regions. Thus, the semi-crystalline polymers have true melting temperatures (Tm) at which the ordered phase turns to disordered phase, whereas the amorphous regions soften over a temperature range known as the glass transition (Tg). It should be noted that amorphous polymers do not possess the melting point, but all polymers possess the glass transition temperature.
The polymer melting point Tm is increased if the double bonds, aromatic groups, bulky or large side groups are present in the polymer chain, because they restrict the flexibility of the chain. The branching of chains causes the reduction of melting Point, as defects are produced because of the branching.
Fig 1:Melting point and glass transition temperature of polymer
T A B L E: Glass Transition Temperatures of Some Polymers
|
Polymer |
Tg (∘C) |
|
Polytetrafluoroethylene |
−97 |
|
Polypropylene (isotactic) |
+100 |
|
Polystyrene |
+100 |
|
Poly(methylmethacrylate) (atactic) |
+105 |
|
Nylon 6,6 |
+57 |
|
Polyethylene (LDPE) |
−120 |
|
Polyethylene (HDPE) |
−90 |
|
Polypropylene (atactic) |
−18 |
|
Polycarbonate |
+150 |
|
Poly(vinyl acetate) (PVAc) |
+28 |
|
Polyester(PET) |
+69 |
|
Poly(vinyl alcohol) (PVA) |
+85 |
|
Poly(vinyl chloride) (PVC) |
+87 |
chains can move easily, then the glassy state can be converted to the rubbery state at lower temperature, that is, the glass transition temperature is lower. If somehow the mobility of the chains is restricted, then the glassy state is more stable, and it is difficult to break the restriction causing the immobility of the polymer chains at the lower temperature, because more energy is required to make the chains free. Thus, in this case, the glass transition temperature is raised.
Factors Affecting the Glass Transition Temperature:
The glass transition temperature depends on the mobility and flexibility (ease of the chain segment to rotate along the chain backbone) of the polymeric chains. If the polymeric
I. IntermolecularForces : Strong intermolecular forces cause higherTg.For example, PVC (Tg = 80 ∘C) has stronger intermolecular forces than polypropylene (Tg = −18 ∘C) because of the dipole–dipole forces from the C—Cl bond.
II.Chain Stiffness : The presence of the stiffening groups (such as amide, sul-fone, carbonyl, p-phenylene etc.) in the polymer chain reduces the flexibility of the chain, leading to higher glass transition temperature.
For example : polyethyleneterephthalete is stiffer than polyethylene adipate due to the presence of benzene ring (see Fig. 1.1). Therefore, Tg value is higher for polyethyleneterephthalate.
III.Cross-Linking : The cross-links between chains restrict rotational motion and raise the glass transition temperature. Hence, higher cross-linked molecule will show higher Tg than that with lower cross-linked molecule.
IV. Pendant groups : The presence of pendent group can change the glass transition temperature.
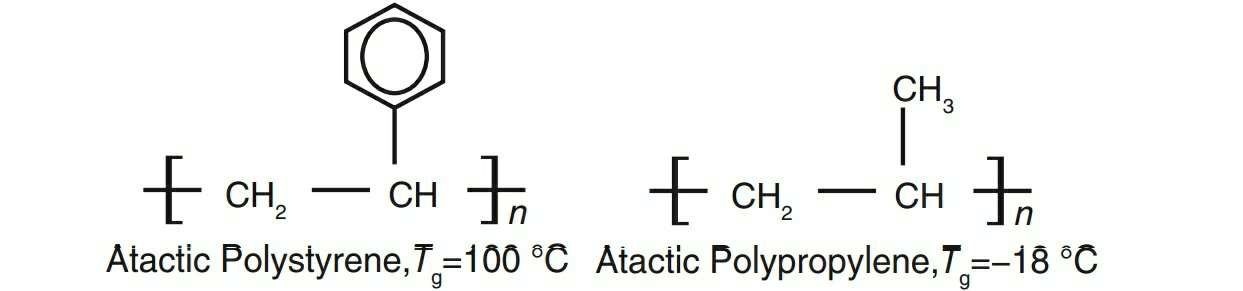
1 . Bulky pendant groups: the presence of bulky pendant group, such as a benzene ring, can restrict rotational freedom, leading to higher glass transition temper-ature. As in polystyrene, the presence of benzene ring increases the Tg (see Fig. A1.6). In polypropylene, there is no benzene ring that leads to lower Tg value (Fig. 1.2).
2 . Flexible pendant groups: the presence of flexible pendant groups, forexample, aliphatic chains, limits the packing of the chains and hence increases.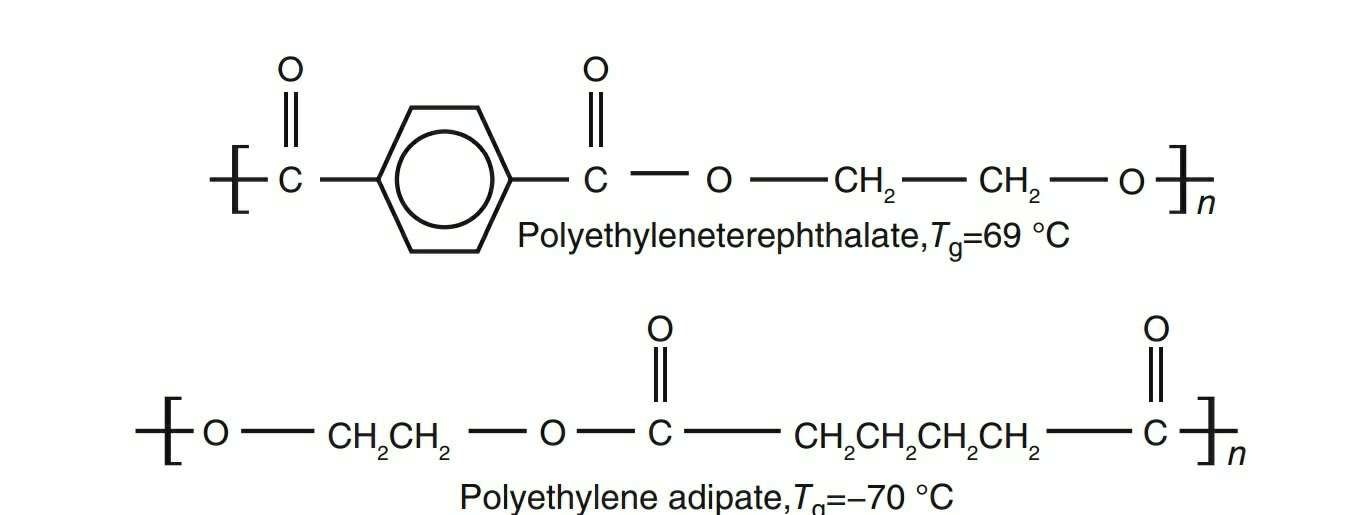
Figure 1.1. Presence of benzene in polyethyleneterephthalete (top molecular chain) makesit stiffer (hence higher Tg) than polyethylene adipate (bottom molecular chain).
Figure 1.2. Role of bulky pendant groups in affecting glass transition temperature.
Figure 1.3. Role of flexible pendant groups in affecting glass transition temperature.
the rotational motion, tending to less Tg value. In polybutylmethacrylate, the presence of large aliphatic chain reduces the Tg value when compared with that of polymethylmethacrylate (Fig. 1.3).
V. Plasticizers : Plasticizers are low molecular weight and non-volatile mate-rials added to polymers to increase their chain flexibility. They reduce the intermolecular cohesive forces between the polymer chains, which in turn decrease Tg.
VI. Molecular Weight : The glass transition temperature is also affected by the molecular weight of the polymer (Fig. A1.8). Tg is increased with the molecular weight. The molecular weight is related to the glass transition temperature by the Fox–Flory Equation:
Tg=Tg,∞ ̶ (K/Mn) (Fox-Flory Equation)
where Tg,∞ is the glass transition temperature at the molecular weight of infinity, and K is the empirical parameter called Fox–Flory parameter related to the free volume inside the polymer. It is observed that Tg is increased up to the molec-ular weight of approximately 20 000, and after this limit, the Tg is not affected appreciably.
Fig : Variation of glass transition temperature with molecular weight
(1234)