Dyeing of Cotton with Vat dye | Part 01
Vat dye
Vat dye is one of the oldest types of dye. It is mainly suitable for cellulosic fibre for best overall fastness properties. Because of the popularity of blue jeans, indigo is still one of the most important dye in the world in the present time. Natural indigo was extracted by fermenting Indigofera tinctoria leaves. But today , Indigo is synthesized from manufactured intermediates.
Indigofera tinctoria
Why called vat dye :
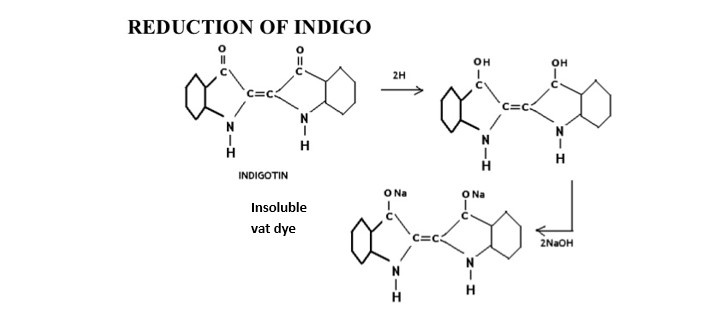
The word vat means “vessel”. They are water insoluble. So it is reduced by fermentation in wooden vessel. The vat dyes are naturally obtained coloring materials from the ancient time and kept into wooden vat and make soluble in vat by the process of fermentation- so it is called vat dyes. They can’t be used directly & requires vatting. They are insoluble in water but become soluble from by vatting process. The process of converting insoluble vat dye by strong reducing agent in called vatting process. This converted, water soluble pigment is called leuco from & they have substantivity for cotton.
Vat / Vessel
Properties
- Vat dye are insoluble & can’t be directly applied to textile material. Before dyeing they need vatting.
- Vat dyes are insoluble in water, solubilised by treatment with caustic soda and reducing agent, usually hyposulphite, the resulting leuco compounds have affinity for textile fiber, on exposure to air leuco compound impregnated fiber re-oxidises to the insoluble parent dye.
- Vat dyes mainly belong to indigoid and anthra quinoid classes and are characterized by high fastness, specially anthraquinoids
- Mainly used or cellulosic fibre but for protein fibre pH should be control properly.
- Most valuable for dyeing and printing cotton, wool and silk.
- After dyeing , the dyes are entrapped inside the fibre & can’t come out during soaping & other treatment. So wet fastness is very good around 4-5 .
- Most of the vat dye are extremely fast to light. So used in curtain.
- Applied in alkaline condition pH 12-14
- Rubbing fastness is not good.
- Various shade is found
- Dyeing process is difficult & different such as sulphure or indigo dyeing process.
- Costly
- Particle size is very small. So poor rubbing fastness.
VAT DYES ARE COMMONLY USED FOR
- Superior Quality Shirting Material
- Military Uniforms
- Furnishings
- Curtain,
- Toweling
- Denim Etc.
Classification:
According to their application process:
Vat dyeing conditions can vary widely in terms of temperature and the amount of salt and alkali required, depending on the nature of the dye applied. Based on temperature, amount of caustic soda, hydrosulphite and salt, used in dyeing, vat dyes can be classified into 3 main groups
1. The 1N (indanthrene normal) group
- IN dyes (N = normal) are highly substantive
- vatting temperatures (60*C) and dyeing temperatures (60*C).
- no salt is added to the dyebath
- because of the high substantivity of the leuco dyes for cotton;
2. The IW (indanthrene Warm) group
- IW dyes (W = warm) have higher affinity
- requires the use of moderate concentrated NaOH and
- lower vatting (50*C) and dyeing temperatures (50*C).
- the leuco forms of these dyes have moderate substantivity for cotton
- some addition of salt is needed during dyeing to aid exhaustion;
3. The IK group
- IK dyes (I = Indanthren, K = cold) have low affinity,
- only need a low concentration of NaOH with
- low vatting (40*C) and dyeing temperatures (20*C).
- these dyes have low substantivity for cotton and
- need considerable salt for good dyebath exhaustion.
Some have amide groups that would be hydrolysed under the vatting and dyeing consitions used for IN and IW dyes.There are special processes for some black vat dyes that require an oxidative aftertreatment to develop the full black colour.
Table compares the characteristics of these three types of vat dye . the required concentrations of hydrose ,caustic soda and salt increase with increasing amounts of dye in the bath and with increasing liquor ratio.
|
|
Condition |
IN |
IW |
IK |
|
1. |
NaOH conc. (g/L) |
4.5-7.5 |
2.0-4.0 |
1.5-2.5 |
|
2. |
NaCl conc. (g/L) |
0 |
5-25 |
10-40 |
|
3. |
Na2S2O4 conc. (g/L) |
1.5-10 |
0.4-6.25 |
0.4-4.5 |
|
4. |
Dyeing temp (C) |
50-60 |
45-50 |
20-25 |
|
5. |
Vatting temp (C) |
50-60 |
45-50 |
40 |
On basis on chemical structure:
On the basis of chemical structure vat dyes are 2 types.
- anthraquinonoid
- indigoid vat dyes.
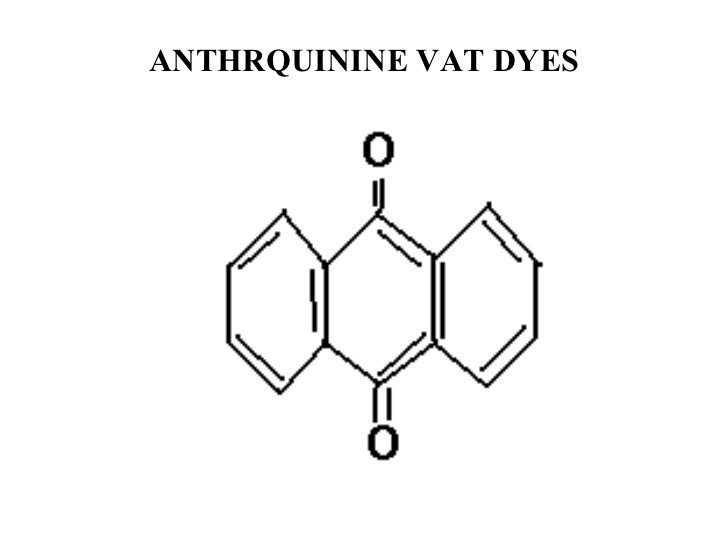
Anthraquinonoid:
In anthraquinonoid , one or more quinine ( >C=O) groups act as chromophores & groups such as hydroxy (–OH) alkylamino (–NHR or NR2) benzamido etc are auxochromes.
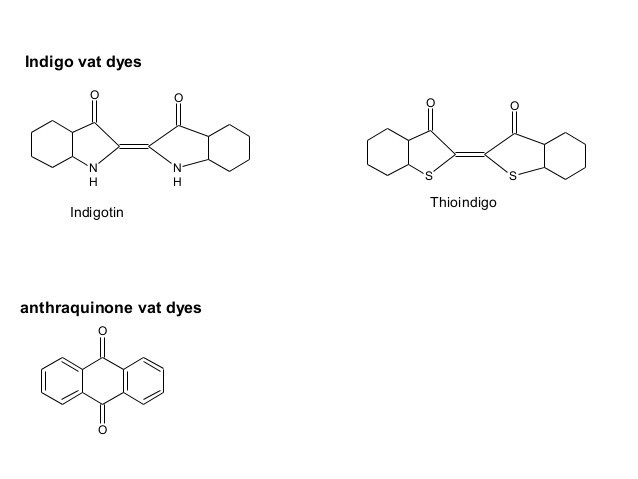
Indigoid :
Indigoid dyes contain (–C-C=C-C–) as the chromophore & (-NH-, -S- as auxochromes. They can be further divided into 4 sub class
- Indigo & its derivatives & homologues
- Thioindigo & its derivatives & homologues
- Indole thianaphthene dyes
- Miscellanious
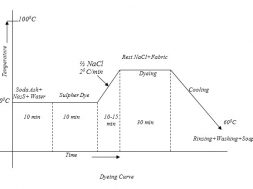
Dyeing of cotton with vat dye
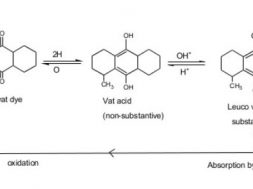
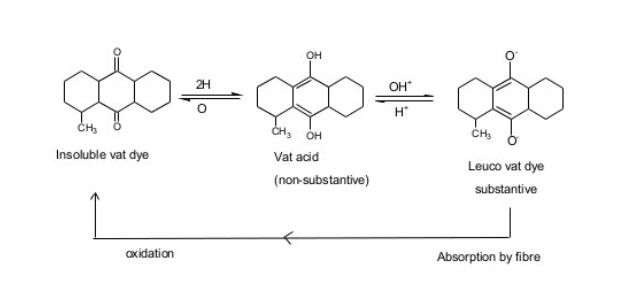
vat dyes are water insoluble pigment. So vatting is done for making it solubilized form. Its application inverts reduction to water soluble leuco compound, then dyeing is done& after that re-oxidation of leuco dye in the fibre to soluble pigments. There are basic 4 steps for vat dyeing. They are——-
1. Vatting
The step in which the reduction of the dyestuff into its leuco-form takes place is called vatting. It is the process of converting insoluble dye to soluble vat dye . they are reduced to soluble leuco compound & this process is called vatting.
Vat dyes are generally more difficult to reduce than sulphur dyes. Various reducing agents are used. Sodium dithionite (hydrosulphite) is still the most widely employed for vatting.
In this stage insoluble vat dye is reduced to produce weak acidic leuco from. A strong reducing agent dihydrosulphite, Na2S2O4.2H2O or hydrose is used for vatting. Vatting is carried out in strong alkali condition.
Decomposition of hydros occours rapidly in acidic solution .so during dyeing material should not be over exposed to air.
2. Dyeing:
Absorbtion of leuco compound by the cotton during dyeing. The vatted dye ( leuco form) are substantive to the cellulose material. Dyeing is carried out at lower temperature. To achieve adequate exhaustion an electrolyte is added to the dye liquor & temperature may be increase from 20-60 C & agitated for 15 min the goods are totally immersed to prevent premature oxidation.
3. Re oxidation of vat:
After absorption by the fibre, the dye in its soluble leuco form is converted to the original pigment by oxidation. This process is carried out in the course of wet treatment (washing) by addition of oxidants such as hydrogen peroxide, perborate to the liquor or exposed to open air.
In this stage unfixed dye deposited on the surface are washed off. The material in weakly alkaline liquor with a detergent at boiling temperature. This soap treatment is not only aimed at removing pigment particles, but also allows the crystallisation of amorphous dye particles, which gives the material the final shade and the fastness properties typical of vat dyes
5. Recipe
Vat dye 1%
Wetting agent 1.5 g/L
Sequestering agent 0.75 g/L
Caustic 4%
Hydrose 3%
Salt 20 g/L
M:L 1:10
Temp 60-100C
(14386)