Collagen: Changing the Course of the Future
Collagen: Changing the Course of the Future
Muh. Amdadul Hoque , Md. Iftekhar Mahmood
Department of Fabric Engineering
A famous American architect and inventor R. Buckminster said, “You never change things by fighting the existing reality. To change something, build a new model that makes the existing model obsolete.” It is in this spirit that the predictions are made. New science can produce products beyond our present perception. Collagen fiber is that name that made a dream into reality.
Collagen is one of the body’s important natural resources and a component of skin tissue and connective tissue. Its application as a wound dressing and surgical material is vastly promising.
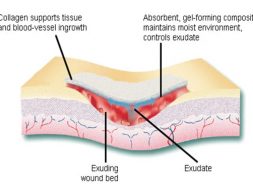
Traditional wound dressing such as plasters, bandages, gauze, lint etc. are ineffective due to their excessive drainage and becomes adherent to the wound surface which makes it painful to remove. These dressings are also easily infected, creating discomfort and irritation, become hard, unease in application and unnatural. Over the last 30 years, there has been a shifting from traditional wound dressings towards those advanced dressing that aim to optimize the wound healing environment. Modern wound dressing have been developed to facilitate the function of the wound rather than just to cover it. These dressings are focused to keep the wound from dehydration and promote healing. Collagen fiber is making its mark in this field for its versatile characteristics which facilitates wound healing process.
Well, the uses of collagen did not start in the modern era. The first use of collagen was found to be more than 8000 years old. But then it was used as glue. Egyptians used collagen as adhesive materials in their paintings and wooden furniture. Real scientific discovery about its basic structure and its huge application field was in 1930’s. Since then many famous scientists and Nobel laureates made their contribution in its development. Research and development about its application in the advanced medical uses began in the 1980’s.
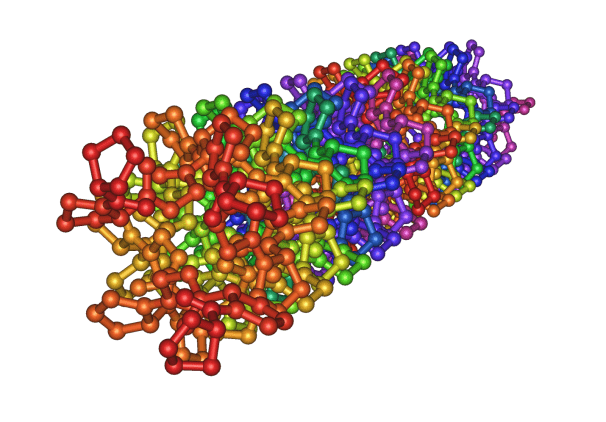
Fig: Crystal structure of the collagen triple helix
Collagen is a common protein and a key component of the extracellular matrix (ECM) in the living (human or animal) body. As a structural protein, collagen is essential to create the body’s physical structure and as an extracellular matrix it acts as a supporting framework over which our cells are arranged. The collagen molecule is composed of three intertwined peptide chains and forms a triple-helical structure. This triple-helical structure of collagen arises from an unusual abundance of three amino acids: glycine, proline and hydroxyproline each performing a precise function. Collagen is a rigid protein 300 nm in length, 1.5 nm in diameter, and approximately 300 kilodaltons (kDa) in molecular weight. The entire molecule consists of about 3,000 amino acids. The triple-helical collagen molecules pack together side by side and form fibrils with a diameter of 50-200nm. The collagen molecules in the fibrils displaced one another by about 65nm. Covalent aldol cross-links form between two adjacent collagen molecules. These cross-links stabilize the side-by-side packing of collagen molecules and generate a strong fibril.
To date 29 types of collagen have been identified. Over 90% of the collagen in the body is of type I-IV; with the most abundant being Type I. Type I and III are important for wound healing. In a wound healing process a large number of things happen quickly in a series which includes- gathering of platelet, inflammation, rapid increase in fibroblast, cell contraction, formation of new blood vessels and restoration of epithelium cells which ultimately lead to scar formation and wound remodeling. In case of normal wound healing in one of these healing stages, a chronic wound is stalled which usually occurs during the inflammatory phase and is related to higher level of matrix metalloproteinase (MMPs) in the wound. MMPs are attracted to the wound and break down unhealthy extracellular matrix (ECM). This leads to new tissue formation. However, if MMPs are present in higher level for a long time, it causes destruction of healthy ECM and increase wound size. Then an alternative method is needed for wound management, which suggests the role of collagen dressing.
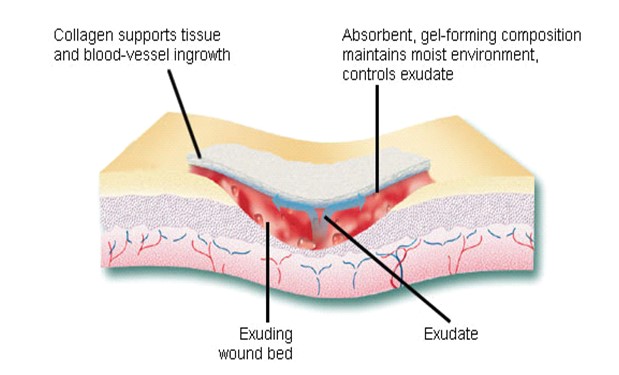
Collagen fiber plays an important role in each of these phases of wound healing. It attracts cells such as fibroblasts and keratinocytes to the wound. This encourages the removal of damaged tissue, formation of new blood vessels and restoration of epithelium cells. Collagen dressings stimulate new tissue growth and encourage the deposition and organization of newly formed collagen fibers and granulation tissue in the wound bed. These dressings chemically bind to matrix metalloproteinase’s (MMPs) found in the extracellular fluid of wounds. MMPs normally attack and break down collagen. Wound dressing containing collagen gives MMPs an alternative collagen source, leaving the body’s natural collagen available for normal wound healing.
Fig: Schematic of wound healing mechanism of collagen
In summary, collagen performs these functions in wound healing-
- Guides fibroblasts which migrate along a connective tissue matrix.
- Attracts fibrogenic cells for healing.
- Creates formation of fibrillar structures.
- Guides new collagen deposition and capillary growth.
- Keeps blood within a damaged blood vessel.
Collagen dressings are suitable in various wound treatment such as bed sores, minor burns, foot ulcers, chronic wounds, large open cuts etc. It is highly effective in-
- Partial and full thickness wounds.
- Wounds with minimal to heavy exudates.
- Skin grafts and skin donation sites.
- Second-degree burns.
- Granulating or necrotic wounds.
- Chronic non healing wounds.
Common source of collagen fiber is from bovine, porcine, equine, or avian sources. Most of the medical collagen is derived from young beef cattle, which is purified in order to make it non-antigenic. It may contain ingredients, such as alginates and cellulose derivatives that can enhance absorbency, flexibility, and comfort, and help maintain a moist wound environment.
Collagen fiber has excellent biocompatibility which makes it popular choice for using as suture. They are as strong as silk and are biodegradable and can readily be accepted by body because of their low immunogenicity. Main features of collagen fiber are- high tensile strength, excellent biocompatibility, non-antigenic.
Scaffolds with nano-fibrous architecture have been produced by the electro-spinning of chitosan followed by imbibing of collagen solution. This scaffold maintains similar structural and functional attributes of native extracellular matrix which makes it applicable in skin tissue engineering. Collagen fiber is used in cosmetic surgery for reconstruction of bone and a wide variety of dental, orthopedic, and surgical purposes. It is widely used in bone grafting because of its certain structure and strength.
A fibrous connective tissue called Tendon, which connects muscle to bone. These are made of closely packed fibrous collagen. The tendons in the foot are highly complex and intricate. Tendon injuries are common in athletes. As the collagen fiber technology has traditionally lacked the ability to create fibers that can be produced continually and controlled, Tendon injuries are notoriously difficult to heal. Currently a team led by Prof. Dr. Mohsen Miraftab at University of Bolton is working on a tendon healing method which can provide a more effective treatment to the damaged tendon. The team has discovered a unique technique to spin collagen fibers, which can be interwoven into the damaged tendon, offering a natural scaffolding support on which cells can grow successfully. The team is pioneering a highly specialized technique, which produces a continuous, single fine thread, developing a strong structure with controllable flexibility and on which cells can flourish.
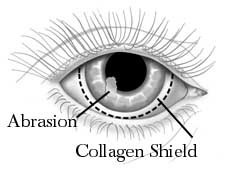
Since collagen is a natural, widely available protein involved in the support and protection of vital structures, many researchers have tried to use extraneous collagen to protect the surface of the eye in a variety of disease states. The pioneer in this research is Dr. Svyatoslav Fyodorov of the Soviet Union who was able to extract collagen from porcine sclera and shape it in the form of a contact lens that can easily be placed on the surface of the eye. Early experience with his collagen “contact lens” showed that it was able to protect the ocular surface and also that it was dissolvable. It seemed that naturally occurring enzymes in the tear film caused the collagen contact lens to dissolve over a period of time within 24 to 72 hours.
Fig: Collagen as contact lens
A number of laboratory and clinical studies are beginning to show that the drug concentrations released from the collagen shield are high enough to be effectively used in treating a variety of diseases including infections and inflammation of the ocular surface.
Along with all of its fascinating advantages, it comes with some problems. Particularly when collagen is obtained from bovine or porcine, it transmits severe foot and mouth diseases. Collagen derived from animal always contains the risk of zoonosis such as Bovine Spongiform Encephalopathy (BSE) and there is always the concern of high production cost. So the scientists have been looking for an alternative source and they have turned to the ocean. Collagen derived from Blue Tilapia fish is the most promising source of fish collagen. Fish collagen is considered to be a novel biomaterial for cell and tissue culture. A team of scientists from American Chemical Society has developed a wound dressing from Tilapia skin collagen sponge and electro spun nano-fibers. They used it to cover rats wound. The rats with the nano-fiber dressing healed faster than those without it. It is found that the collagen nano-fibers stimulated the skin regeneration rapidly and effectively. Furthermore, fish collagen was not likely to cause any immune reaction. So it is a good candidate for clinical use. The nano/micro fibrous fish collagen scaffolds were fabricated through self-assembly owing to its amphipathic nature. This collagen scaffold is highly potential in cell and tissue engineering. Despite its huge potential, there is comparatively less on-going research in this field.
Bibliography
- https://en.wikipedia.org/wiki/Collagen.
- http://www.woundsresearch.com/content/a-review-collagen-and-collagen-based-wound-dressings.
- http://pubs.acs.org/doi/pdf/10.1021/i360061a018.
- http://www.wounds-uk.com/made-easy/collagen-dressings-made-easy&print.
- https://www.researchgate.net/publication/236093564_Recent_advances_on_the_development_of_wound_dressings_for_diabetic_foot_ulcer_treatment-A_review.
- http://online.liebertpub.com/doi/abs/10.1089/wound.2015.0660?journalCode=wound&.
(602)